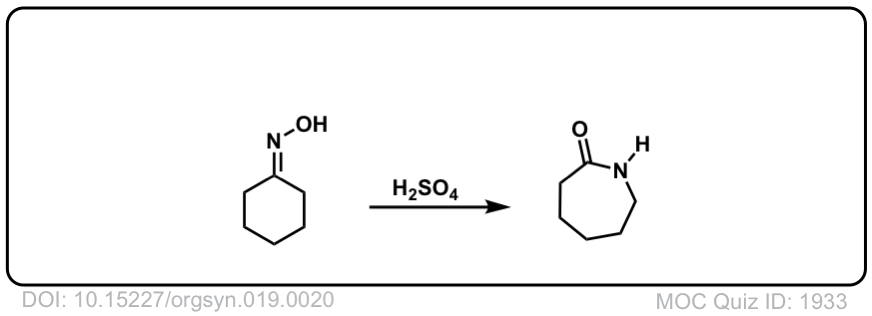
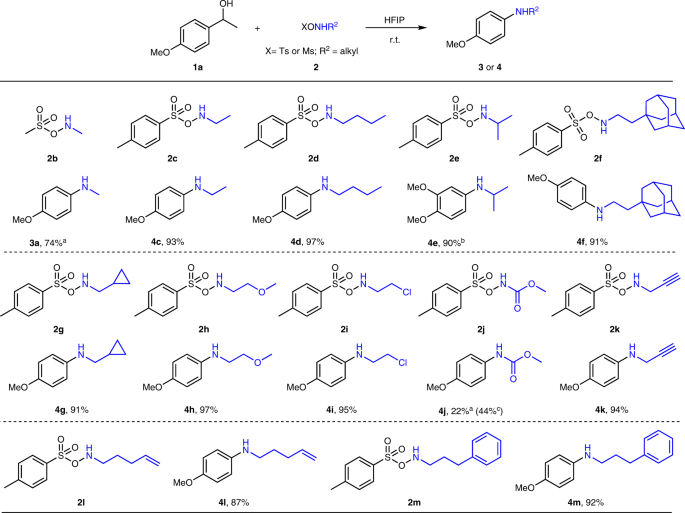
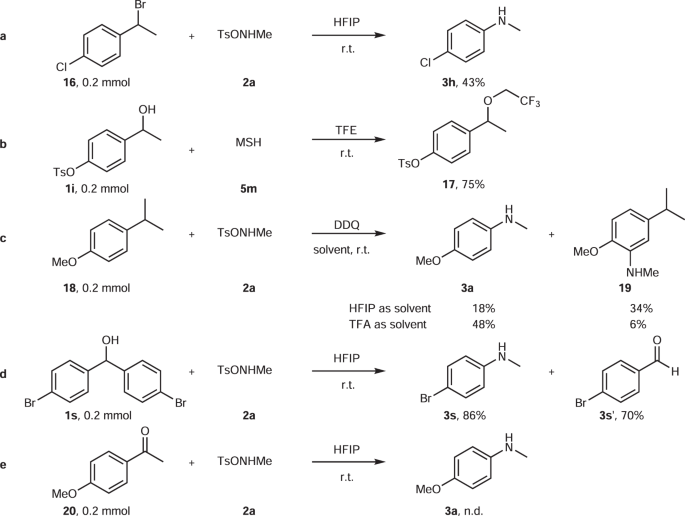
Mechanistic Insight into Self-Propagation of Organo-Mediated Beckmann Rearrangement: A Combined Experimental and Computational Study | The Journal of Organic Chemistry
![Synthesis of 6-alkyl analogues of the 1-azabicyclo[4.3.0]nonan-2-one system by a strategy of geminal acylation and Beckmann rearrangement - Journal of the Chemical Society, Perkin Transactions 1 (RSC Publishing) DOI:10.1039/B108164K Synthesis of 6-alkyl analogues of the 1-azabicyclo[4.3.0]nonan-2-one system by a strategy of geminal acylation and Beckmann rearrangement - Journal of the Chemical Society, Perkin Transactions 1 (RSC Publishing) DOI:10.1039/B108164K](https://pubs.rsc.org/image/article/2002/P1/b108164k/b108164k-s3.gif)
Synthesis of 6-alkyl analogues of the 1-azabicyclo[4.3.0]nonan-2-one system by a strategy of geminal acylation and Beckmann rearrangement - Journal of the Chemical Society, Perkin Transactions 1 (RSC Publishing) DOI:10.1039/B108164K

The Mechanochemical Beckmann Rearrangement: An Eco-efficient “Cut-and-Paste” Strategy to Design the “Good Old Amide Bond” | ACS Sustainable Chemistry & Engineering

Direct synthesis of secondary amides from ketones through Beckmann rearrangement using O-(mesitylsulfonyl)hydroxylamine - ScienceDirect

The Mechanochemical Beckmann Rearrangement: An Eco-efficient “Cut-and-Paste” Strategy to Design the “Good Old Amide Bond” | ACS Sustainable Chemistry & Engineering

Scope and Mechanism of a True Organocatalytic Beckmann Rearrangement with a Boronic Acid/Perfluoropinacol System under Ambient Conditions | Journal of the American Chemical Society

Dichloroimidazolidinedione-Activated Beckmann Rearrangement of Ketoximes for Accessing Amides and Lactams | The Journal of Organic Chemistry

Zinc(II)-Catalyzed Synthesis of Secondary Amides from Ketones via Beckmann Rearrangement Using Hydroxylamine-O-sulfonic Acid in Aqueous Media

Dichloroimidazolidinedione-Activated Beckmann Rearrangement of Ketoximes for Accessing Amides and Lactams | The Journal of Organic Chemistry

Cu(OTf)2-catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA). - Abstract - Europe PMC

Direct synthesis of secondary amides from ketones through Beckmann rearrangement using O-(mesitylsulfonyl)hydroxylamine - ScienceDirect

Beckmann Rearrangement of Ketoximes to Lactams by Triphosphazene Catalyst | The Journal of Organic Chemistry

Zinc(II)-Catalyzed Synthesis of Secondary Amides from Ketones via Beckmann Rearrangement Using Hydroxylamine-O-sulfonic Acid in Aqueous Media,Synthesis - X-MOL
![PDF] Cu(OTf)2-Catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA) | Semantic Scholar PDF] Cu(OTf)2-Catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA) | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0615b0928d07b0fb565394b813ca3929843b6cea/2-Table1-1.png)
PDF] Cu(OTf)2-Catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA) | Semantic Scholar